Increasing offerings and capacity to meet industry demand, CSP Labs is on the cutting edge of genotype and pathology testing technology.
Offering both genotype and pathology testing, Pleasant Grove, California’s CSP Labs, is one of only a few third-party laboratories in the country to offer both options to the fruit and vegetable industry.
“We are using a variety of different chemistries right now;” says CSP lab manager, Hilary Cartwright, “it is really about working with our clients to determine what works best for them, what they are looking for and what crop we are testing.”
Using polymerase chain reaction (PCR), in which a single DNA sequence is replicated millions of times, CSP Lab’s performs genotype testing to determine the genetic pattern and markers of a sample. The tests provide their customers with variety identification, hybrid purity and provides the framework for marker assisted selection.
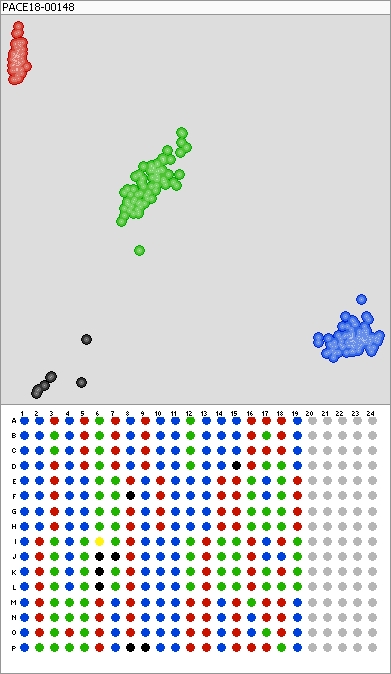
“Genotyping is a much cheaper and faster way of verifying plant and seed purity to confirm that it is the right variety and what percentage of the seeds are hybrid,” Cartwright says.
Today, CSP boasts a vast genotype database of fruit, nut-tree and vegetable varieties, with over 500 unique varieties of strawberries catalogued.
“By offering both genotyping and pathology, CSP has positioned itself as a ‘one-stop shop’, for the mid-size companies we cater to.” says Nilesh Maharaj, lab director for CSP Labs.
Maharaj shares that with the growth of its genotyping laboratory, CSP has continued to expand services to the vegetable and fruit industry, offering industry leading technology.
“We have followed industry demand, focusing more on KASP™ technology because it is much more cost effective and robust; we can analyze more information in a shorter period of time and the chemistry is much more stable than previous methods,” he says.
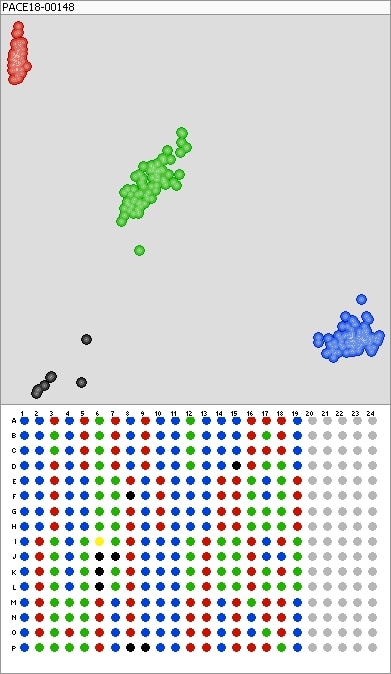
KASP™ technology provides a unique allele-specific PCR that provides precise assessment of SNPs (single nucleotide polymorphisms) and InDels (insertions and deletions) across a large array of genomic DNA samples. Using KASP™ tests can be performed in a shorter window of time with less manpower than other testing technologies — translating into a more cost effective test for both CSP and its industry customers.
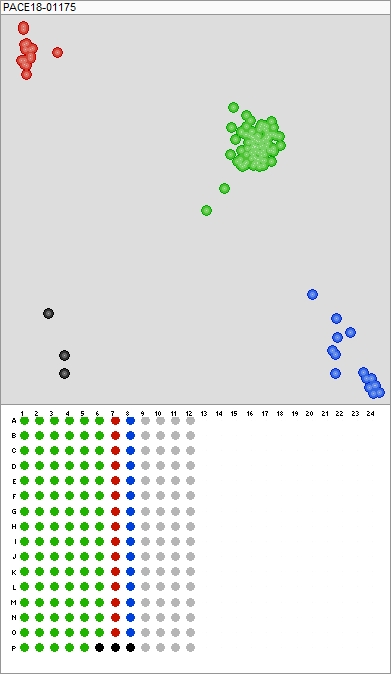
“Prior to these types of technologies (KASP™), industry standard was to grow-out the samples to phenotypically observe that they were all the same,” says Cartwright. “KASP™ provides results in hours, not days; we can analyze the results or a client [using the correct software] can analyze. The test is robust and easier to perform. It is an ideal test to screen a small number of markers within a large number of samples.”
To support the increased demand for KASP™ technology testing, CSP has increased its PCR capacity and doubled the number of employees in the lab; effectively doubling the capacity of its lab through manpower, space and equipment.
“The cost of this technology [KASP™] has been steadily decreasing and that has opened opportunities for CSP to reach new customers, as well as providing existing customers the opportunity to send in more samples,” says Maharaj.