Soybean cyst nematode is one of the world’s most destructive soybean pests. How can we begin to combat it?
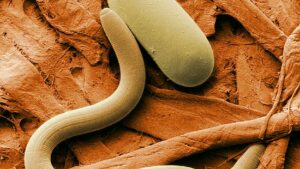
Widely described as the world’s most destructive soybean pest, soybean cyst nematode (SCN) is estimated to cause losses exceeding $1 billion each year in the United States and Canada alone.
What makes this pest so hard to manage? Cysts containing eggs can survive dormant in the soil for 10 or more years. Once a field is infected with SCN, there’s no way to rid them; growers must manage them.
Plants infected with high numbers of SCN have poorly developed root systems that cannot effectively use nutrients and water. Plants that show visible SCN damage might appear slightly yellow and stunted. More frequently, however, infected plants have no apparent symptoms except for yield loss.
Easy Answers
Breeders identified PI 88788 as a resistance line in 1962, but it was not available to farmers until the 1980s. For 20-plus years, more than 95% of all SCN-resistant soybean varieties in the Midwest included resistance from the PI 88788 gene.
PI 88788 is still effective; however, researchers and farmers are seeing resistance build. All it takes is a few resistant individuals to create a problem.
“Some may think that the resistant varieties cause SCN to mutate, but that’s not the case,” explains Greg Tylka, an Iowa State University professor of plant pathology and microbiology. “There is always the possibility, but overwhelmingly, SCN resistance is the result of natural selection driven by
PI 88788.
“When using the same herbicide- or nematode- resistant gene or antibiotic over and over, the small minority in a population will eventually become the majority.”
As the proportion of resistant nematodes in the soil increases, soybean yields decline. Yield data from 15 years of Iowa variety trials, summarized by the SCN Coalition, indicate as much as a 14 bushel-per-acre average yield loss for varieties with PI 88788 resistance grown in SCN fields infested.
The fatal flaw is that virtually all SCN-resistant varieties have the same resistance genes. Tylka says there’s a 97% chance, at least in Iowa and likely throughout the Midwest, that the resistance in newly released varieties is from PI 88788.”
He says the remaining 3% is nearly all from Peking, another SCN-resistant line.
“It’s surprising that the PI 88788 resistance has done as well as it has for as long as it has,” says Brian Diers, a University of Illinois professor and soybean breeder. “However, when we look at SCN samples from fields, we generally find that SCN is infecting PI 88788 more readily than in the past.
“It is not that resistance from PI 88788 has completely broken down and it is not working. When nematode samples from farmer’s fields are tested, we often see that the roots of PI 88788 have about 30-40% as many nematodes as a susceptible check. On the one hand, this means that resistance is breaking down but on the other hand, it means resistance is still 60-70% effective.”
Still, Tylka, like others is worried about how we will deal with the decrease in the effectiveness of the resistance currently deployed in varieties.

“If nothing changes, there’s only one outcome: more yield loss caused by nematodes,” he says.
When Tylka teaches SCN management, he compares managing it to that of high blood pressure.
“Anyone with high blood pressure knows there is not one single pill to control their condition,” Tylka says. “In addition to medication, there are also all sorts of behavioral changes that work together to manage this chronic health problem.”
The first step to manage SCN is to take a soil test to assess the density of SCN infestation. Growers are often unaware they have a problem.
Growing a resistant variety is the prescribed pill. The other critical thing is to rotate resistant varieties. This is difficult because there are not many alternatives.
Tylka says researchers hope this will change as breeders become more successful in developing high-yielding varieties with new sources of resistance.
Crop rotation with a nonhost crop such as corn, oats or alfalfa is one of the behavioral changes needed to control SCN.
The SCN Coalition reports the largest decline in SCN numbers occurs following the first season a nonhost crop is grown. Seed treatments should also be considered.
Seed treatments can help reduce SCN reproduction and/or increase soybean yields in SCN infested fields or they may have no effect. Results vary among the different seed treatment products, between growing seasons and soil environments.
“At this point nobody can tell a farmer at the beginning of the season when or where a particular product will or will not work,” Tylka says. “That is the nature of biology. We thought we had a home run back in the 1990s with PI 88788 but times change and nature overcomes. Nature is the ultimate arbitrator.”
Using cover crops to manage SCN is a new concept. Work coming out of Europe indicates cover crops could reduce nematode numbers. As such, several U.S. universities are studying the role of cover crops and looking at why some do, and others do not, affect SCN populations.
“In one field, a crop of annual rye, for example, might reduce SCN numbers while having no effect in another field,” Tylka says. “There is much interest in cover crops and SCN.”
Simply put, the more measures a farmer uses to control SCN or any pest, the less likely any individual management tactic is going to suffer decreased performance.
Future Prospects
In February 2019, Iowa State University researchers published the fully assembled SCN genome, which could lead to the development of better pest management strategies.
“The soybean cyst nematode genome sequence reveals a diversity of mechanisms that give rise to virulence genes,” says lead author Thomas Baum, an Iowa State University professor and chair of plant pathology and microbiology. “It provides new insights into the SCN’s biology and sheds light onto the mystery underlying complex host-parasite interactions.”
Funding for the research was provided by the North Central Soybean Research Program (NCSRP) with money from the soybean checkoff and the National Science Foundation Center for Arthropod Management Technologies and its industry partners.
The NCSRP in collaboration with the University of Missouri, the University of Illinois and Iowa State University is in the fourth year of a multi-year project to develop an integrated approach to enhance the durability of SCN resistance for long term strategic SCN management. The new SCN genome will provide critical information in the team’s efforts to develop new soybean varieties with SCN resistance.
“The widespread lack of genetic diversity in SCN resistance in soybean has significantly reduced the effectiveness of current sources of resistance,” says Melissa Mitchum, project leader and a molecular nematologist at the University of Missouri. “We have two major research challenges that, when successfully achieved, will enable us to develop more efficient management practices for this pest.”

Additional team members include Brian Diers and Andrew Scaboo, plant breeders; Greg Tylka, an Extension field nematologist, Thomas Baum, molecular nematologist, and Andrew Severin and Matt Hudson, both informatics specialists to work on the computational side.
The project’s objectives are to first understand the genetics of the nematode, then understand the genetics of the plant. Finally, the researchers plan to marry those two objectives to develop new types of genetic resistance and better diagnostic tools to apply resistance more strategically. Mitchum says they also hope to identify genetic vulnerabilities within the nematode that will allow them to engineer novel forms of resistance.
“This project is focused on breaking the SCN resistance cycle and working with plant breeders to diversify and stack soybean’s genetic resistance,” Mitchum says. “Diers‚Äô group has already stacked other types of resistance on top of PI 88788 resistance.
“Other breeders are identifying additional sources of new resistance and getting those into varieties.”
Nematologists are simultaneously working with the breeders to put those varieties into rotation schemes that would make sense for farmers. The project looks at taking some of these gene stacks breeders have developed and incorporating them into a rotation scheme to see how it impacts nematode populations. The scheme calls on farmers to more strategically rotate resistance to not only preserve the current source but also to better manage virulent nematode populations in the field.
Researchers have completed a greenhouse study that rotated Peking resistance with the stacked resistance Diers developed which includes the wild soybean Glycine soja resistance stacked on top of the PI 88788 resistance. The second phase of this study is a three-year field trial beginning this spring to evaluate what happens when moved to fields in Missouri, Iowa and Illinois.
On top of the reference genome, Mitchum’s lab is working to develop a series of adapted nematode populations. One will be highly adapted to the PI 88788 type of resistance, another population that is highly developed to the Peking-type of resistance and another population that is highly adapted to the Glycine soja-type of resistance. The team will then sequence the genomes of those populations and compare them to a population with very few individuals that can grow on a resistant variety. She hopes to pinpoint the genes in the nematode that allow it to overcome these sources of resistance.
Speed Matters
Right now, if farmers want to know if they have a nematode population that can overcome a current source of resistance, they must send a soil sample to a lab for an SCN HG type test. It is a labor-intensive analysis that takes a month to complete. With a molecular diagnostic tool, a diagnostic lab might be able to extract the nematode from that soil sample and overnight provide a result as to whether or not the nematode population has adapted to any type of soybean resistance.
“We call this precision nematode management,” Mitchum says. “This would allow farmers to have access to different types of genetic resistance and plant varieties that have the most resistance to the nematode population in their field. Maybe a farmer has no SCN presence but if they do get the nematode and they keep planting the same PI 88788 resistance, then we will just be perpetuating the issue.”
As new types of soybean resistance are developed, seed companies will need to take up these other types of resistance and bring them to market. Then farmers will need to know how to rotate them strategically to bring their population levels down.
“Genetic resistance is farmers‚Äô best tool for managing SCN. It works really well when it works,” Mitchum says. “We need to maintain the current sources of resistance and breed high-yielding varieties with new sources and deploy them strategically.