The EU Proposal on certain New Genomic Techniques.
WHY IT MATTERS
In 2018, after a ruling of the European Court of Justice put the latest breeding methods under the burdensome EU GMO legislation, the Volume 5, Issue 3 edition of European Seed (Aug. 2018) was titled: “Rest in Peace Plant Breeding Innovation”. Since the EU proposed new regulations for certain new genomic techniques (NGTs) on 5 July 2023, faith in the resurrection of plant breeding innovation in the EU may just start to return. The European plant breeding and seed production sector welcomes that the Commission proposal finally recognizes the need for a differentiated regulatory approach to certain plants and products from NGTs from the GMO legislation.
The proposal of the EU Commission was long awaited. Since 2008, discussions on the rules applicable to new plant breeding techniques (as they were called at the time) have been ongoing. However, their regulatory status was never clarified by the Commission, despite lots of requests by academia and businesses alike. It was only after the European Court of Justice ruling, confirming the GMO status of plants resulting from newer breeding methods like genome editing, that political discussion gained speed and intensity. Scientists and breeders complained, EU Member States foresaw enforcement issues, and an increasing number of countries around the world took a more differentiated regulatory approach. Meanwhile, the plants developed with NGTs were clearly showing their potential to successfully deal with adverse weather conditions and climate change, increase resistance against pests and diseases and/or carry healthy nutritional traits. So, these plants clearly proved their potential to contribute to the objectives of the European Green deal and its Farm to Fork strategy. This finally led to the policy initiative of the EU Commission which, after detailed stakeholder consultations, studies by the Joint Research Centre and the European Food Safety Authority, and an extensive impact assessment, resulted in the proposal published on 5 July 2023.
Conventional-like Plants and Products
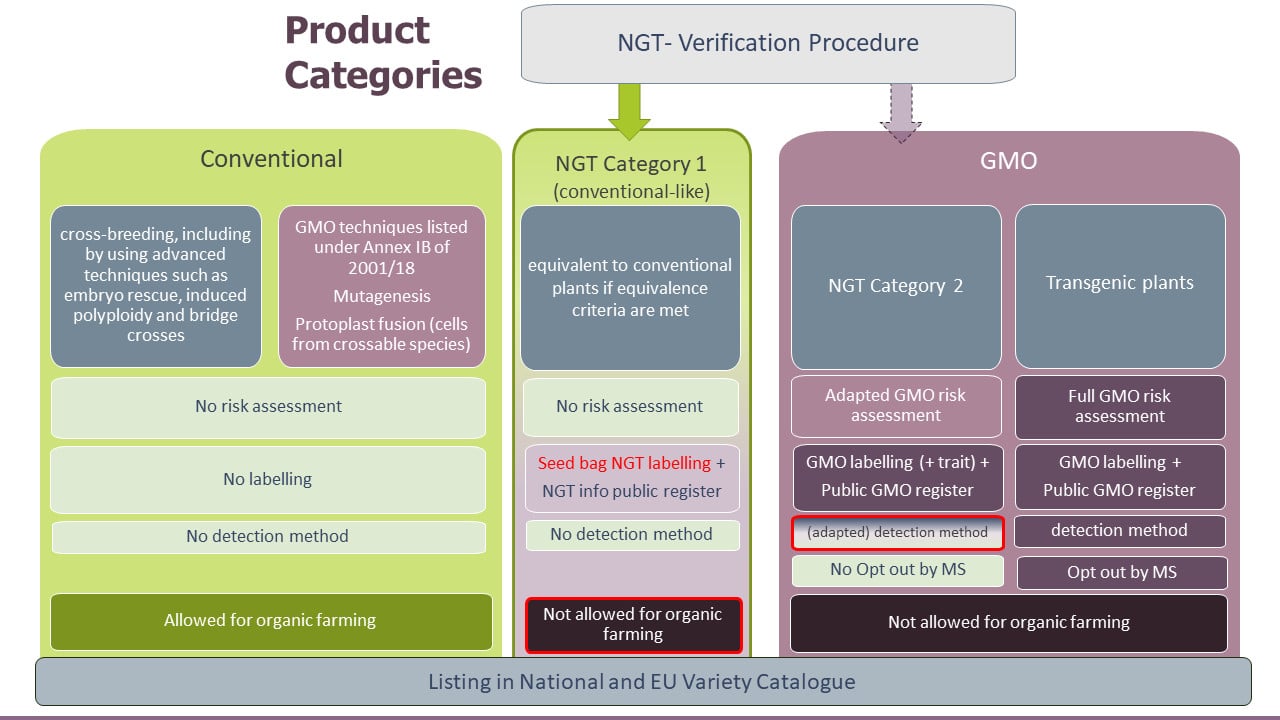
The proposal for a regulation foresees a verification procedure for plants and products resulting from targeted mutagenesis and cisgenesis is as follows:

If the compliance of NGT plants with certain equivalence criteria is confirmed by authorities, these plants and their products would be considered conventional-like (Category 1), to which the rules of the EU GMO legislation do not apply. A summary of the verification decision would be published in a database together with an identification number for the NGT plant and product. Seeds of Category 1 NGT plants would need to be labelled as Category 1 NGT plants and respective information would be included in the EU Common Catalogue of varieties. Category 1 NGT plants would remain subject to any regulatory framework that applies to conventionally bred plants.
If NGT plants and products do not meet the equivalence criteria, they would be considered Category 2 and are subject to an adapted GMO risk assessment as well as GMO detection, traceability, and labelling requirements. Although Category 1 plants and products would be deemed “conventional-like”, both categories of NGT plants and products would not be allowed for organic farming.
The regulatory approach taken by the EU Commission does not exclude NGT plants and products from the GMO legislation but is considered a “lex specialis” with regard to the EU GMO legislation. It introduces specific provisions for NGT plants and products and with this creates a distinct product category for conventional-like NGT plants and products, including specifically blocking organic farming to utilize verified conventional-like NGT plants.
The verification process would be carried out by national authorities, making it accessible to all organizations, including small- and medium-sized companies. Verification would be based on molecular data that confirms the absence of foreign genes and shows that the product meets the Equivalence Criteria for Category 1. It would not include a risk assessment and NGT Category 1 plants and products would not require a defined detection method. Nevertheless, other Member States and the Commission would be able to provide comments to the draft verification report of a national competent authority. This would lead to a delay in the verification process and an assessment by the European Food Safety Authority (EFSA) and the Commission as well as a voting by member states before the Commission takes a final decision on the verification.
Authorization process for Category 2 NGT plants and products
The authorization process for Category 2 NGT plants and products would be based on molecular data to confirm absence of foreign genes, molecular data on genetic modifications and could require additional data for composition, phenotype, toxicity/allergenicity if the problem formulation gives rise to a plausible risk hypothesis. Category 2 plants would also require a detection method that complies with the requirements for GMO detection methods. In cases where it would not be feasible to provide an analytical method that detects, identifies and quantifies, if duly justified by the notifier or the applicant, the modalities to comply with analytical method requirements could be adapted. The labelling of category 2 NGT products as GMO could be complemented with information on the trait conferred by the genetic modification.
Regulatory incentives
The proposal also foresees regulatory incentives to potential notifiers or applicants for category 2 NGT plants and products containing traits with the potential to contribute to a sustainable agri-food system, in order to steer the development of category 2 NGT plants towards such traits. Traits that would be included in regulatory incentives include yield, tolerance/resistance to biotic stresses, tolerance/resistance to abiotic stresses, resource use efficiency (such as water and nutrients), characteristics that enhance the sustainability of storage, processing and distribution; improved quality or nutritional characteristics and the reduced need for external inputs, such as plant protection products and fertilisers. Herbicide tolerance traits would not be applicable for regulatory incentives (like regulatory advice or waiver of fees for SMEs).
The opt-out option for Member States to not allow cultivation of EU-approved GMOs would not be applicable to Category 2 NGT plants.
A first take of the EU seed sector on the proposal
The EU seed and breeding sector reiterates that any NGT-derived plant which is verified as conventional-like should also be subject to the same regulatory framework as conventional breeding products. Any additional requirements would be discriminatory and unjustified.
Based on this principle, it is inconsistent that conventional-like NGT plants would be considered GMOs for organic farming under the proposed regulations (Art 5(2)). This would create additional hurdles for organic breeding and farming as well as legal uncertainty, both for breeding companies and for traders and would unfairly prohibit organic farming from benefitting from any of the NGT-derived product innovations in plant breeding that we expect in the years to come.
Conventional and organic agriculture have coexisted without specific coexistence rules or limits. Creating new barriers would result in numerous measures being taken by farmers and would increase costs across the agri-food value chains.
Euroseeds supports transparency and consumer choice by making information about the use of NGTs publicly available (i.e., in the EU’s common catalogue of plant varieties or other public databases). We consider seed bag labelling for verified conventional-like NGT plants as problematic and discriminatory.
Together, the prohibition for organic production and the seed bag labelling provisions create a third category of plant products between conventional ones and GMOs. This is not in line with the approaches taken in other countries and might create trade issues. The Commission could have taken a much clearer regulatory approach by exempting verified Category 1 NGT plants from Directive 2001/18, e.g., by adding them to Annex IB and with that being exempted from the GMO legislation consistent with random mutagenesis.
A conservative approach with the potential of politicization
We regret that the Commission took a very conservative approach regarding the equivalence criteria in view of the limited numbers of genetic changes and the exception of endogenous-gene interruptions for a plant to be covered by the equivalency criteria for Cat 1. This discriminates polyploid crops which include multiple copies of the same gene and for which each modification would be counted separately and against knock-in/knock-out improvements (of disease-resistance for example). A diploid crop with only two copies of a gene (alleles) could still benefit from being classified in Category 1 while the same trait in a polyploid crop with several copies of the same gene would lead to a Category 2 product.
Even though the EFSA concluded that off-target effects are of the same nature as changes occurring by conventional breeding, including random mutagenesis, the Commission proposes to consider off-target changes at predictable locations under the limit of 20 genetic modifications. There is a risk that the identification of these off-targets might discriminate crops (specifically smaller crops) for which no reference genome sequence is available. This would severely limit the diversity of applications of NGTs and the diversity of crops.
Euroseeds has always highlighted that any GMO-light approach (Category 2) is unworkable, specifically for SMEs. Therefore, for Category 2 plants that are covered by such a GMO-light regime, the incentive mechanisms for Category 2 authorizations will be ineffective.
We also have serious doubts about enforceability in regard to detection and traceability requirements for NGT products of Category 2 for which no or only an adapted detection method can be developed, that can unequivocally identify the regulated product. One of the conclusions of the Commission Study on NGTs in 2021 was that the current GMO legislation is difficult to enforce due to lack of distinction from conventional products, specifically in view of unauthorized products. If for imports, it is not possible to identify unauthorized NGTs with Category 2 changes, it is discriminatory to require GM traceability and labelling of such products in the EU. Any Cat 2 plant/product that cannot be distinguished from a conventional product should therefore be considered as a Category 1 NGT plant/product to not again run into enforcement issues.
The Verification Process for Category 1 Plants must provide legal clarity and should be foreseeable and reliable. The EU seed and breeding sector welcomes that national competent authorities are the responsible bodies for conducting the verification procedure. This increases accessibility for SMEs. Nevertheless, the criteria in Annex 1 on which the verification is built need further clarification to allow a common understanding between national competent authorities and, with this, legal certainty for developers.
Breeding companies invest up to 20 per cent of their turnover in research and development. They rely on legal certainty for their investments. The verification process should therefore be based on clear criteria and the scientific expertise of member states’ competent authorities. Any interventions by member states or the Commission should be limited to scientifically justified “comments” only and not be based on political considerations.
What’s next?
The Commission proposal is only the first, but of course important, step in the legislative procedure. The proposal will now be discussed by the European Parliament and the Council to develop a text that finally needs to be adopted by all three EU institutions. Having in mind that the EU has elections in June 2024, the likelihood that the Parliament will conclude its discussions before then is rather limited, but not impossible. After an adoption of the regulation, the EU Commission would then need to draft and adopt implementing acts that make the regulation operational by outlining details of the procedures, data requirements etc. Only then would first products be verified, field trialled and marketed under the new regulatory regime.
The resurrection of plant breeding innovation in the EU has been initiated by the Commission proposal for a regulation of certain NGTs. But the gravestone is heavy and requires the full political commitment in Parliament and Council to finally be fully removed and, through this, to unleash the potential of plant breeding innovation to contribute to a competitive, sustainable, and productive EU agriculture.
Equivalence Criteria Proposal
A NGT plant would be considered equivalent to conventional plants when it differs from the recipient/parental plant by no more than 20 genetic modifications of the types referred to in points 1 to 5, in any DNA sequence sharing sequence similarity with the targeted site that can be predicted by bioinformatic tools.
(1) substitution or insertion of no more than 20 nucleotides;
(2) deletion of any number of nucleotides;
(3) on the condition that the genetic modification does not interrupt an endogenous gene:
(a) targeted insertion of a contiguous DNA sequence existing in the breeder’s gene pool;
(b) targeted substitution of an endogenous DNA sequence with a contiguous DNA sequence existing in the breeder’s gene pool;
(4) targeted inversion of a sequence of any number of nucleotides;
(5) any other targeted modification of any size, on the condition that the resulting DNA sequences already occur (possibly with modifications as accepted under points (1) and/or (2)) in a species from the breeders’ gene pool.
Editor’s Note: Petra Jorasch is Manager Plant Breeding Innovation Advocacy at Euroseeds